Scuba Theory 101
TheScubaDirectory ⋅ June 19, 2024
Mastering the natural laws that govern scuba diving is essential for every diver. Understanding these principles, along with the limits and effects on our bodies and the environment, ensures safety and enhances the overall diving experience. Dive into the science behind the sport to keep yourself and the ocean safe.
Breathing Underwater
As a new recreational diver, you will likely be breathing compressed air from your dive cylinder. As you advance in your dive career, you may continue your training to dive with different gas mixes such as Nitrox or Trimix. For the purpose of this guide, we will focus on breathing from common air-filled cylinders.
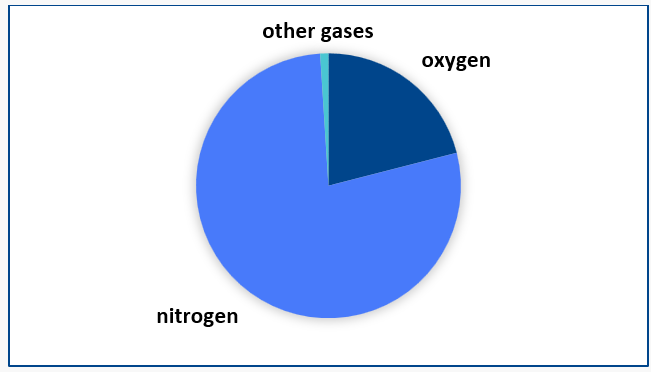
A common misconception is that scuba tanks are filled with 100% oxygen. In reality, an air-filled tank typically consists of 21% oxygen, 78% nitrogen, and 1% of other gases.
Nitrogen is a tasteless, odorless, and colorless gas and even though it is a major component of the earth’s atmosphere, us humans do not use it physiologically. Although our bodies do not use it, Nitrogen has certain impacts and risks when breathed under pressure such as decompression sickness and nitrogen narcosis.
Oxygen is also a tasteless, odorless, and colorless gas. The right volume of oxygen is critical as too much or too little can create risks and problems for divers.
The Natural Laws of Diving
It is important to understand the natural laws that pertain to scuba diving. In general, dive equipment is designed to take account of the laws of pressure.
Boyle’s Law
Boyle's Law is particularly relevant to scuba diving because it describes the relationship between pressure and volume of a gas at a constant temperature. As a diver descends underwater, the pressure increases and this affects the volume of the gas in the diver's body and equipment.
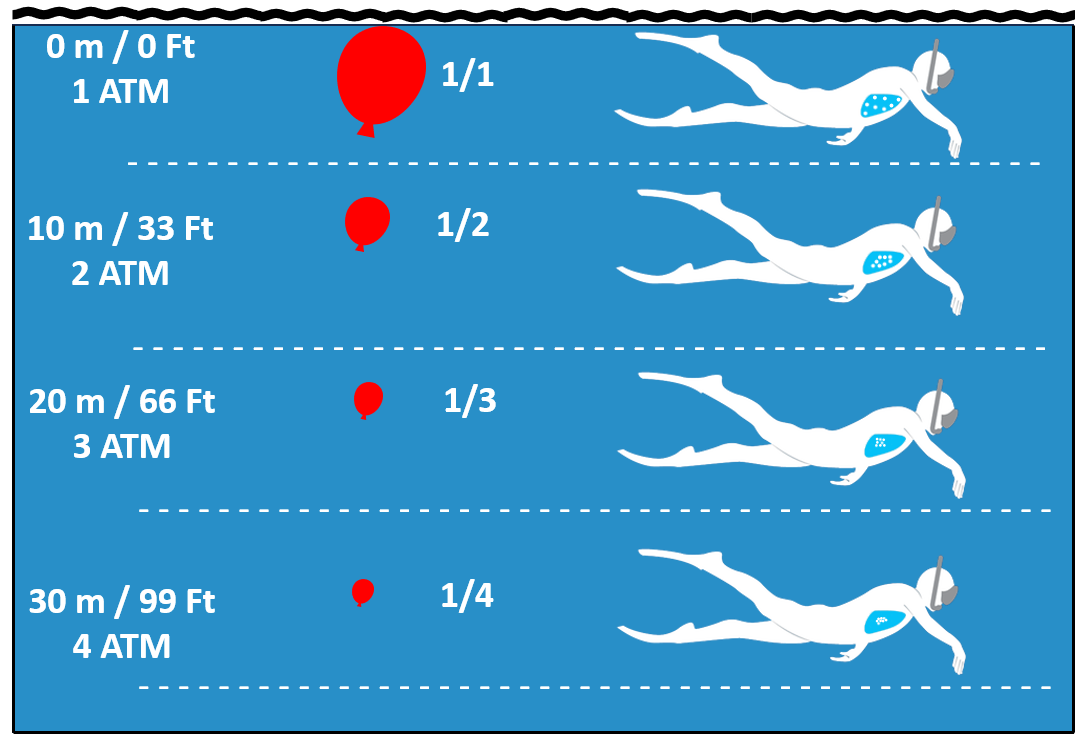
At the surface, the surrounding air pressure is fairly constant. We refer to this standard pressure as 1 atmosphere (ATM). 10 meters (33 feet) of sea water exerts the same amount of pressure as the 1 ATM. Therefore, we can add 1 ATM pressure for every 10 meters (33 feet) you descend. In the chart below, you can see that the balloon and air in the diver’s lungs compress more with each ATM they descend to.

Why is Boyle’s Law important to scuba diving?
As a diver descends underwater, the pressure on their body increases and the air spaces (lungs, ears, sinuses) are compressed. As the diver ascends, the pressure decreases and the air in these spaces expands.
The number one rule of scuba diving is to never hold your breath. This is because holding your breath stops you from being able to equalize the air space in your lungs. If you were to ascend while holding your breath, then the air would expand and you could acquire serious injury to your lungs.
It is important to ascend slowly when scuba diving to avoid decompression sickness. While you scuba dive, your body takes on nitrogen from the air in the tank, ascending slowly gives the nitrogen time to off-gas safely. Ascending fast can cause the nitrogen to form bubbles, which expand and lead to decompression sickness.
The Natural Law of Buoyancy
In scuba diving, the natural law of buoyancy is closely tied to Archimedes' Principle, which governs how objects behave when submerged in a fluid. Buoyancy control is a fundamental skill for scuba divers and understanding the principles of buoyancy helps divers maintain proper depth, control ascent and descent, conserve energy, and enhance safety underwater.
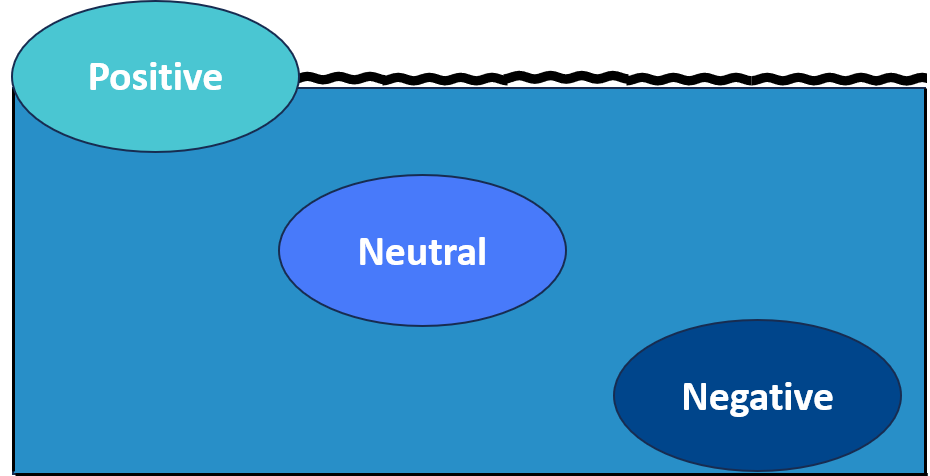
Positive Buoyancy and Negative Buoyancy
- Positive buoyancy occurs when the buoyant force acting on an object is greater than its weight, causing it to float.
- Negative buoyancy occurs when the weight of an object is greater than the buoyant force, causing it to sink.
- Neutral buoyancy is achieved when the weight of the object equals the buoyant force, allowing it to hover at a constant depth.
Scuba divers use Buoyancy Control Devices (BCDs) to manage their buoyancy. By adding or releasing air from the BCD, divers can increase or decrease their buoyancy and achieve neutral buoyancy. Mastering your buoyancy is a skill you will practice during your Open Water Training and into your dive career. It is one of the key skills that will lead to happy and successful diving.


